VAERS data released Friday by the Centers for Disease Control and Prevention included a total of 1,103,893 reports of adverse events from all age groups following COVID vaccines, including 23,615 deaths and 188,135 serious injuries between Dec. 14, 2020, and Feb. 4, 2022.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,103,893 reports of adverse events following COVID vaccines were submitted between Dec. 14, 2020, and Feb. 4, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 23,615 reports of deaths — an increase of 466 over the previous week — and 188,135 reports of serious injuries, including deaths, during the same time period — up 4,824 compared with the previous week.
Excluding “foreign reports” to VAERS, 753,482 adverse events, including 10,747 deaths and 70,746 serious injuries, were reported in the U.S. between Dec. 14, 2020, and Feb. 4, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 10,747 U.S. deaths reported as of Feb. 4, 18% occurred within 24 hours of vaccination, 23% occurred within 48 hours of vaccination and 60% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 541.5 million COVID vaccine doses had been administered as of Feb. 4, including 318 million doses of Pfizer, 205 million doses of Moderna and 18 million doses of Johnson & Johnson (J&J).
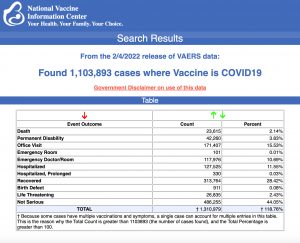
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed. Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to Feb. 4, 2022, for 5- to 11-year-olds show:
- 7,724 adverse events, including 170 rated as serious and 3 reported deaths.
The most recent death involves a 7-year-old girl (VAERS I.D. 1975356) from Minnesota who died 11 days after receiving her first dose of Pfizer’s COVID vaccine when she was found unresponsive by her mother. An autopsy is pending.
- 16 reports of myocarditis and pericarditis (heart inflammation).
- 29 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to Feb. 4, 2022, for 12- to 17-year-olds show:
- 28,793 adverse events, including 1,651 rated as serious and 38 reported deaths.
The most recent deaths involve a 13-year-old male (VAERS I.D. 2042005) from an unidentified state who died from a sudden heart attack seven months after receiving his second dose of Moderna, and a 17-year-old female from an unidentified state (VAERS I.D. 2039111) who died after receiving her first dose of Moderna. Medical information was limited and it is unknown if an autopsy was performed in either case.
- 68 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases attributed to Pfizer’s vaccine.
- 629 reports of myocarditis and pericarditis with 617 cases attributed to Pfizer’s vaccine.
- 155 reports of blood clotting disorders, with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to Feb. 4, 2022, for all age groups combined, show:
- 19% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 72.6.
- As of Feb. 4, 5,038 pregnant women reported adverse events related to COVID vaccines, including 1,615 reports of miscarriage or premature birth.
- Of the 3,531 cases of Bell’s Palsy reported, 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 858 reports of Guillain-Barré syndrome (GBS), with 40% of cases attributed to Pfizer, 30% to Moderna and 28% to J&J.
- 2,316 reports of anaphylaxis where the reaction was life-threatening, required treatment or resulted in death.
- 1,576 reports of myocardial infarction.
- 12,981 reports of blood clotting disorders in the U.S. Of those, 5,780 reports were attributed to Pfizer, 4,627 reports to Moderna and 2,527 reports to J&J.
- 3,950 cases of myocarditis and pericarditis with 2,427 cases attributed to Pfizer, 1,343 cases to Moderna and 169 cases to J&J’s COVID vaccine.
Pfizer and BioNTech delay request to authorize vaccine for children under 5
Pfizer and BioNTech announced today they are delaying their request to the FDA to authorize the Pfizer-BioNTech COVID vaccine for children under five years old, citing not enough data on the efficacy of a third dose.
The FDA said its advisory panel meeting scheduled for next week will be postponed. Pfizer was originally expected to publish an analysis of its data today.
Pfizer said it will wait for its data on a three-dose series of the vaccine — expected in April — because it believes three doses “may provide a higher level of protection in this age group.”
Pfizer said in December 2021 that two doses of its Pfizer-BioNTech vaccine failed to generate a strong immune response during its clinical trial of children ages 2 to 4.
For children aged 6 months to 5, Pfizer’s vaccine has a dosage of 3 micrograms. For children ages 5 to 11, the dosage is 10 micrograms.
Despite the results of its trial, the company asked the FDA this month to authorize these first two doses, with a plan to submit additional data in the coming weeks on a third dose, NBC reported.
As The Defender reported Wednesday, some experts speculate the push to expand the authorization to the younger age group would lay the groundwork for subsequently folding COVID shots into the childhood vaccine schedule — thereby ensuring “liability protection forever.”
6-year-old gets myocarditis, can’t walk, after receiving COVID vaccine
Milo Edberg, 6, has been intubated and hospitalized since receiving his COVID vaccine on Dec. 10, Alpha News reported.
Edberg’s mother, Carrie, said her son was at M Health Fairview’s Masonic Children’s Hospital in Minneapolis, Minnesota for a minor procedure when a doctor recommended he receive the COVID vaccine.
Carrie said she followed the advice of her doctor, who told her the vaccine was “safe and harmless.”
“I went against my gut and said OK, do it,” she said.
Carrie said the evening after receiving the shot, her son was gasping for air. She dialed 911. Edberg was transported back to the hospital, was intubated and diagnosed with myocarditis.
He was “perfectly fine and then he wasn’t,” Carrie said. He was “eating on his own [but] now he can’t even swallow his saliva.”
Doctors have no answers and cannot explain her son’s affliction, Carrie said. They haven’t even been able to provide a timeline for when her son might return home or whether he will regain any quality of life — and they “won’t bring up the vaccine” when discussing Edberg’s situation.
Carrie filed a VAERS report in January and said her son received a 10-15 minute visit from an infectious disease specialist who said they would file a report with the CDC and and Pfizer early in his hospital stay. She has heard nothing since.
The CDC maintains most cases of myocarditis after COVID vaccines are “mild” and patients recover quickly.
Not all doctors agree. As Dr. Steven Pelech of the University of British Columbia explained last August:
“Contrary to what a number of people have said, there is no such thing as ‘mild myocarditis.’ It’s the destruction of the myocytes, the heart cells that contract. When those cells die, they are not replaced in your body and are instead replaced by scar-tissue, which is from fibroblasts — skin cells which don’t have contractile activity …Every time you get an inflammatory response, you lose more of that contractility and have a greater chance of heart attack and other problems later in life.”
A New Zealand writer observed that “mild” clinical manifestations in the present are meaningless for interpreting longer-term risks.
Using magnetic resonance imaging (MRI) scans with gadolinium contrast — capable of showing “damaged heart areas undetectable by any other means” — studies of children and adolescents who developed myocarditis following COVID vaccination revealed, in the vast majority, a “potentially poor prognosis despite the heart seeming to have returned to normal.”
Kansas woman died from allergic reaction to Moderna’s COVID vaccine
Jeanie Evans, 68, of Effingham, Kansas, died of “anaphylaxis due to COVID-19 vaccination,” according to her autopsy report acquired by the Topeka Capital-Journal.
Evans died March 24, 2021, one day after her first dose of Moderna’s vaccine.
According to the autopsy report, Evans said her airway felt blocked about 15 to 20 minutes after she received her first dose on March 23, 2021. She was taken by ground ambulance at 5:21 p.m. to Stormont-Vail hospital, where she died at 11:55 a.m. the next day.
Evans had a medical history of hypertension, environmental allergies, allergic disorders and reactive airway disease. She previously experienced an anaphylactic reaction to the drug Albuterol, the report said.
Colt Umphenour, one of Evans’ sons, said the family plans to file a lawsuit.
Denmark officials see no reason to continue COVID vaccine program
Health authorities in Denmark announced Friday they are considering “winding down the entire general vaccination program later in the spring.”
According to the Associated Press, officials see no reason to administer a booster dose to children or a fourth shot to residents at risk of severe COVID.
The Danish Health Authority said in a statement the third wave of COVID was waning “due to the large population immunity,” and the country can cope with increasing infection without getting serious illness.
The agency said it would continue to follow the epidemic closely should there be a fourth spring wave or new worrying variants.
Denmark ended most of its pandemic restrictions earlier this month after officials said they no longer considered COVID “a socially critical disease.”
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.


